Intestine Toxicology Services Overview
Validated 3-D models, bespoke analytics, actionable output
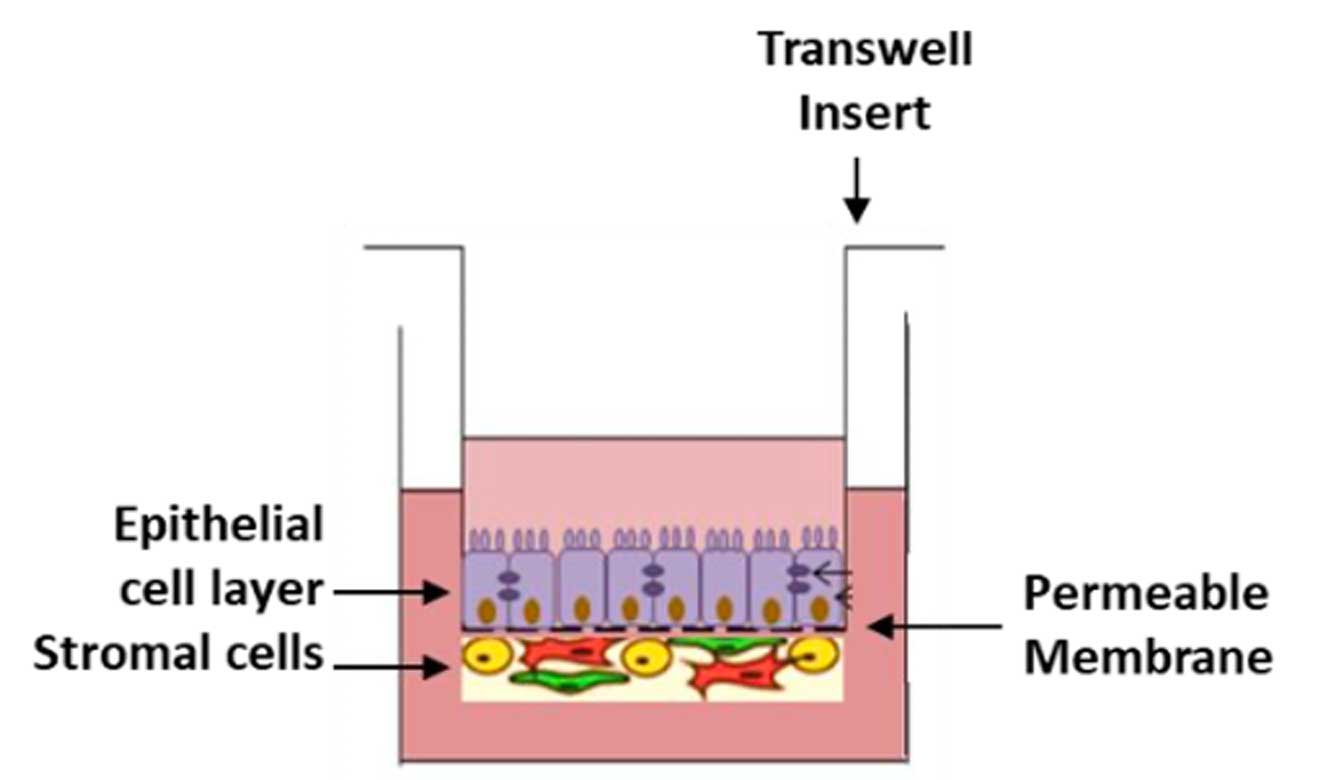
Drug-induced gastrointestinal (GI) toxicity and AEs/SEs, particularly diarrhea, are a leading cause of clinical product development attrition. The NAMkind™ Intestine Model from VivoSim offers a robust, human-relevant solution, combining advanced Transwell-based technology with expert scientific support to help you detect and mitigate GI risk early. Unlike traditional static in vitro screens, our full-service platform provides comprehensive insight through barrier function testing (TEER), cell viability assays (ATP, Alamar Blue), and histological analysis for structural damage. Share your compounds and key questions, and within four weeks, you will receive detailed, clinically relevant results.
Built with human epithelial and key stromal cells such as endothelial, smooth muscle cells, and fibroblasts, the model replicates the intestinal barrier’s complexity to move beyond single-layer cultures, enabling more predictive and translational assessments of drug-induced toxicity. Our scientists collaborate directly with your team, interpreting results, contextualizing risk, and providing strategic recommendations aligned with your development milestones. Whether you are performing early compound screening, validating clinical findings, or probing structure-toxicity relationships, the NAMkind™ Intestine Model delivers clarity and confidence.