Actionable Human-Safety Data
Without the Guesswork
At VivoSim we develop, human-relevant, customizable NAMkind™ 3-D liver and intestinal toxicology models that accelerate lead selection, de-risk compounds selection, and advance promising candidates with confidence.
The problem
Late-stage drug failures waste millions of investment dollars and delay treatments because early toxicology models either miss human-specific liver and intestine toxicity signals or show false signals in animal-derived 2D, 3D, and in vivo models.
The solution
VivoSim Labs delivers NAMkind™ 3D models alongside expert toxicology services, providing human-relevant safety insights before costly animal studies or clinical trials begin. Our species-specific liver and intestine NAMkind™ 3D models clarify cross-species relevance, guiding the selection of the most predictive in vivo animal models and a clear path into the clinic. VivoSim offers bespoke study designs, species-specific variants, and modular assay panels—delivering actionable insights tailored to each client’s timelines and budget.
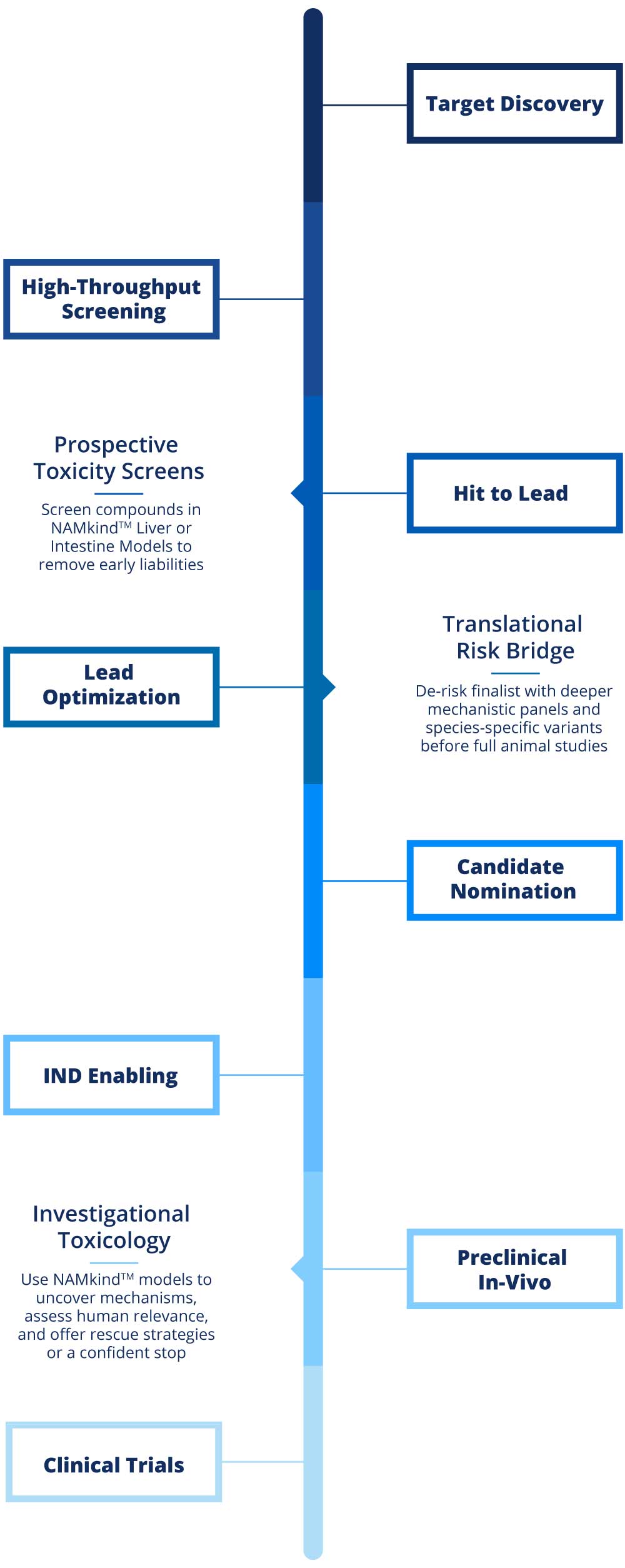
VivoSim Labs anchors its NAMkind™ services at three decision-critical points.
Prospective Screens during Hit-to-Lead and early Lead Optimization. In two weeks, our Liver and Intestine models can screen 20+ compounds, flag early liabilities through core biomarkers, and deliver insights that guides chemistry toward safer compounds
Translational Bridge begins in late Lead Optimization and continues through Candidate Nomination. Here we deploy species-specific models plus optional mechanistic panels to confirm human relevance, fine-tune safety margins, and supply regulatory-ready narratives before and throughout IND-enabling studies
Investigative Toxicology is an on-demand service activated when unexpected findings emerge in animal or human studies. The same NAMkind™ models reproduce or refute the signal, map root-cause pathways and provide clear go, modify or stop recommendations within weeks
Target Discovery
Hit to Lead
Translational
Risk Bridge
De-risk finalist with deeper mechanistic panels and species-specific variants before full animal studies
Candidate Nomination
Preclinical In-Vivo
#192D5D
#284A8E
#255AAF
#2469A8
#2F88F2
#7ABBFC
#93CBF9
#B8DCF4
High Throughput Screening
Prospective
Toxicity Screens
Screen compounds in NAMkindTM Liver or Intestine Models to remove early liabilities
Lead Optimization
IND Enabling
Investigational
Toxicology
Use NAMkindTM models to uncover mechanisms, assess human relevance, and offer rescue strategies or a confident stop
Clinical Trials
What is NAM/NAMkind
To overcome the limitations of traditional toxicity testing, New Approach Methodologies (NAMs) aim to be more informative, faster, more cost-effective. The goal is to reduce or replace animal testing using validated and reliable 3D and tissue-based models.
VivoSim’s NAMkind™ models utilize primary human cells to create micro-liver and micro-intestine organoids that more accurately reflect relevant biological responses than traditional toxicology tests. The NAMkind™ Liver and NAMkind™ Intestine models are specifically designed to help customers identify and overcome toxicology challenges, driving drug advancement in a timely and safe manner.
Comprehensive Assay Portfolio
Core and bespoke assays that translate complex biology into clear safety insight
Histological Assessment
Cell Viability
Epithelial Barrier Integrity
Oxidative Stress
Gene Expression
Hepatocyte Function
Liver Injury
Inflammatory Markers
Histological Assessment
Liver:
Histological assessment in liver models involves microscopic examination of tissue architecture, cell morphology, cell types, and extracellular matrix composition. Liver models are H&E and immunohistochemistry stained for markers such as collagen (fibrosis) and cytokeratin (hepatocyte integrity). These stains hallmark features of liver injury and disease progression. High-resolution imaging allows quantification of tissue health, identification of necrosis or apoptosis, and assessment of multicellular organization, closely mirroring in vivo pathology. This approach is critical for validating disease models and evaluating toxic effects
Intestine:
For intestinal models, histological assessment focuses on the integrity and organization of the epithelial layer, presence of goblet and Paneth cells, and the underlying stromal compartment. Staining for tight junction proteins (e.g., ZO-1, occludin), mucins, and inflammatory markers enables visualization of barrier function and disease-associated changes such as crypt distortion, ulceration, and fibrosis. Histology can distinguish between healthy and diseased states by revealing disrupted architecture and increased collagen deposition. RNA sequencing and multiplexed imaging further enhance the ability to correlate histological features with gene expression and functional outcomes.
Cell Viability
Liver:
Cell viability in liver models is measured using ATP quantification assays, which reflect metabolic activity and overall cell health. Additional markers include LDH release (membrane integrity) and live/dead staining. These assays are essential for screening for drug candidates, as reduced viability correlates with hepatotoxic potential. Viability endpoints are integrated with functional assays (albumin) to provide a holistic view of liver health and response to injury. The platform’s flexible dosing schedule enables assessment of viability over treatment durations exceeding 20 days.
Intestine:
In intestinal models, cell viability is assessed using ATP assays and metabolic indicators such as Alamar Blue reduction. These readouts quantify the proportion of living, metabolically active cells and are sensitive to cytotoxic insults from drugs or inflammatory stimuli. Viability assays are performed alongside barrier integrity and inflammatory markers to distinguish between reversible and irreversible damage. Maintaining high cell viability is crucial for modeling helathy states and evaluating toxic potential of therapeutic interventions.
Epithelial Barrier Integrity
Liver Barrier Integrity:
While the liver is not a classical barrier organ, hepatocyte tight junctions and sinusoidal endothelial integrity are important for maintaining selective permeability and preventing leakage of toxic substances. In vitro, barrier integrity can be inferred from LDH release, albumin secretion, and the maintenance of polarized cell layers. Disruption of these features is indicative of liver injury and compromised function.
Intestinal Barrier Integrity:
Barrier integrity is a defining feature of intestinal models, measured by transepithelial electrical resistance (TEER) and permeability assays. High TEER values indicate intact tight junctions and functional epithelial layers, while drops in TEER signal barrier disruption—a hallmark of IBD, infection, or drug-induced toxicity. Permeability to fluorescent tracers or macromolecules further quantifies barrier function.
Oxidative Stress
Liver:
Oxidative stress in liver models is assessed by measuring glutathione (GSH) levels. GSH depletion is an early indicator of hepatocyte injury.
Intestine:
In intestinal models, oxidative stress is measured using ATP and Alamar Blue quantification and assessment of redox-sensitive gene expression. Oxidative stress contributes to barrier dysfunction, inflammation, and epithelial cell death in drug-induced injury.
Gene Expression
Liver:
Gene expression profiling in liver models utilizes RNA sequencing and qPCR to quantify transcripts associated with hepatocyte function, injury, fibrosis, and inflammation. Key genes include CYP450 enzymes, transporters (BSEP, MRP), albumin, and markers of apoptosis or necrosis. Differential expression analysis reveals drug-induced changes, disease signatures, and therapeutic responses. Integration with histological and functional data enables comprehensive characterization of model fidelity and mechanistic pathways.
Intestine:
For intestinal models, gene expression analysis targets epithelial differentiation markers, tight junction proteins, cytokines, and fibrosis-related genes. RNAseq distinguishes healthy from diseased tissue by identifying upregulation of inflammatory mediators, downregulation of barrier proteins, and altered extracellular matrix components. These data provide molecular validation of model architecture and disease relevance, supporting drug-induced toxicity assessments.
Hepatocyte Function
Hepatocyte (Liver-Specific)
Hepatocytes are the principal functional cells of the liver, responsible for metabolism, detoxification, and protein synthesis. Our models assess hepatocyte health via albumin production, CYP activity, and transporter function. Maintenance of polarized morphology and bile canaliculi formation are indicators of mature hepatocyte phenotype. Drug-induced changes in hepatocyte function are central to DILI prediction and mechanistic toxicology.
Enterocyte (Intestine-Specific)
While hepatocytes are liver-specific, intestinal models focus on enterocytes, goblet cells, and other epithelial subtypes. Enterocyte function is assessed via transporter activitu and barrier maintenance. The interplay between epithelial cells and immune/stromal compartments is critical for modeling disease and drug response.
Liver Injury
Liver:
Liver injury is evaluated using a combination of cell viability, functional assays (ALT/AST, albumin), and histological markers (necrosis, fibrosis). LDH release and ATP depletion are early indicators, while chronic injury manifests as collagen deposition and altered gene expression. Advanced models replicate clinical patterns of injury, enabling mechanistic studies and therapeutic screening. Sensitivity and specificity of injury detection are benchmarked against FDA-classified compounds.
Intestine:
Intestinal injury is characterized by loss of barrier integrity, increased cell death, and upregulation of inflammatory and fibrotic markers. TEER drops, permeability increases, and histological evidence of ulceration or crypt loss are key endpoints. These features are central to modeling chemotherapy-induced mucositis, and drug-induced GI toxicity.
Inflammatory Markers
Liver:
Inflammatory markers in liver models include cytokines (IL-6, TNF-α), chemokines, and immune cell infiltration. Multiplexed assays and immunostaining quantify the inflammatory response to drugs or disease stimuli.
Intestine:
In intestinal models, inflammatory markers encompass cytokines and chemokines (IL6, IL-8, IL-1β, MCP1) and immune cell recruitment. Elevated levels correlate with barrier dysfunction, epithelial cell death, and disease severity in drug-induced injury. Advanced platforms enable dynamic measurement of inflammatory responses, supporting the development of precision medicine approaches.
Publications
October, 2025
Bioprinted 3D Primary Human Intestinal Tissues Model Aspects of Native Physiology and ADME/Tox Functions
Madden LR, Nguyen TV, Garcia‑Mojica S, et al. iScience. 2018;2:156–167. doi: 10.1016/j.isci.2018.03.015
Read more »October, 2025
Development of a Multicellular 3D Intestinal Model Using Human Primary Cells to Identify Novel IBD Therapies
Gervacio S, Dudum R, Aidnick H, et al. Digestive Disease Week 2025, Abstract No. Tu2035
Read more »October, 2025
Identification of JAK Inhibitors as Potential Therapeutics for Inflammatory Bowel Disease Using Human Primary Cell 3D Models of Crohn’s Disease and Ulcerative Colitis
Dudum R, Payton O, Toohey C, et al. Digestive Disease Week 2025, Abstract No. Tu2040
Read more »
